How to Find Limiting Reactant: A Complete Guide
Understanding **how to find limiting reactant** is crucial for anyone studying chemistry. A limiting reactant determines the amount of product that can form in a chemical reaction, influencing both theoretical yield and actual yield calculations. In this guide, we will delve into the **definition of limiting reactant**, outline effective methods for determining it, and provide practical examples that will help illustrate these concepts. Whether you’re a student or a professional looking to enhance your knowledge of **chemical reaction stoichiometry**, this informative piece will serve as a valuable resource.
Understanding the Basics of Limiting Reactants
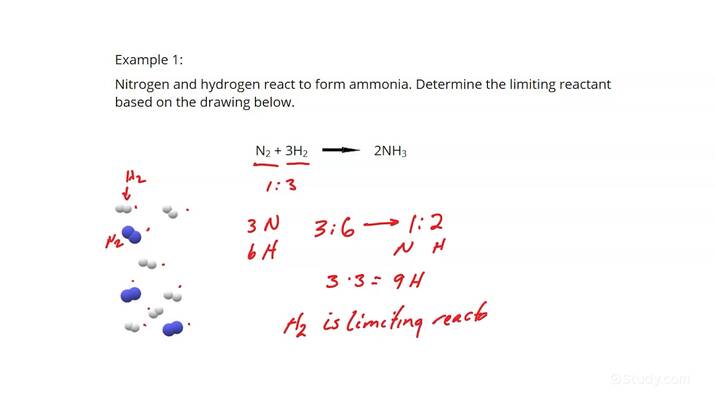
The first step to mastering the concept of limiting reactants is understanding what they are. A **limiting reactant**, or limiting reagent, is the substance that is consumed first in a chemical reaction. Once this reactant is completely used up, the reaction cannot continue, regardless of the quantities of other reactants present. Knowing the ***reactants and products*** in a balanced chemical equation helps in identifying which reactant will limit the reaction. For instance, in the equation \( 2H_2 + O_2 \rightarrow 2H_2O \), if you have more hydrogen than oxygen, oxygen would be the limiting reactant.
Balanced Chemical Equations
To effectively **calculate limiting reactants**, you must start with a properly **balanced chemical equation**. This ensures that the number of atoms for each element is conserved during the reaction. For example, if we consider the reaction mentioned above, balancing the equation shows you exactly how many molecules of each reactant are required for complete consumption. This step is essential before proceeding to more detailed calculations regarding reactants, tendencies, and yields.
Mole Ratios and Stoichiometric Coefficients
Understanding **mole ratios** from the balanced equation is critical. These ratios give insight into the proportion in which reactants combine and products form. For the balanced equation of \(2H_2 + O_2 \rightarrow 2H_2O\), the mole ratio of hydrogen to oxygen is 2:1. If you start with 6 moles of hydrogen and 2 moles of oxygen, you can identify that the oxygen will limit the reaction, ultimately resulting in only producing a specific number of water molecules.
Visualizing Limiting Reactants with Examples
To more effectively illustrate how to identify limiting reactants, let’s consider a **limiting reactant example**. Imagine you’re baking a cake requiring flour and sugar. If your recipe states it needs 2 cups of flour and 1 cup of sugar for one cake, but you have 6 cups of flour and just 2 cups of sugar, sugar becomes the limiting reactant. Here, using the right amounts of each will yield the maximum number of cakes. In terms of chemical calculations, just as in baking, excess reactants don’t affect the yield—but the limiting one does.
Calculating the Limiting Reactant
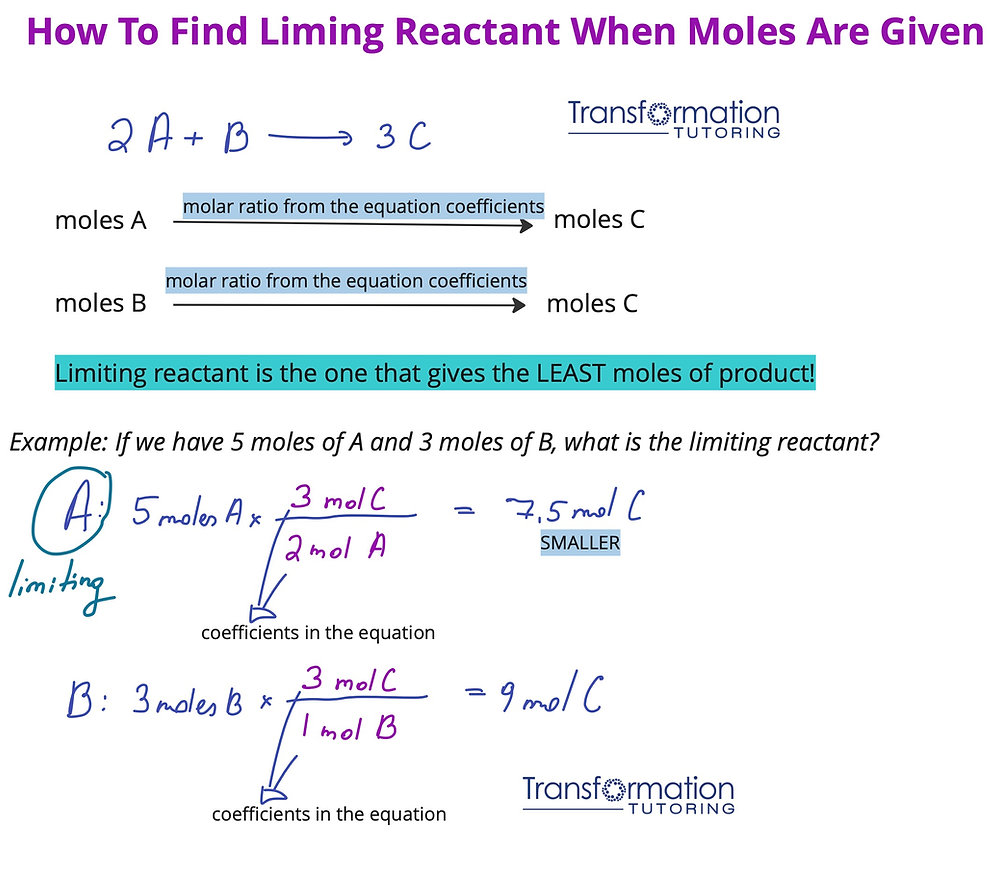
Now that we’ve defined limiting reactants and discussed the fundamentals, let’s explore some methods for **calculating limiting reactants** in your chemical reactions. Proceeding with stoichiometric calculations is essential for accurate results. The following subsections outline steps and examples that will aid in your computation process.
Finding Limiting Reactant Steps
The process to find the limiting reactant involves a few systematic steps:
1. **Write the balanced equation.**
2. **Convert all given information to moles** using molar mass if necessary.
3. **Use mole ratios** derived from the balanced equation to determine how much of each reactant is needed and compare it with what you actually have. If you have 6 moles of one reactant but only require 3 for reaction completion, that reactant is in excess.
4. **Identify the limiting reactant** based on which substance is used up first.
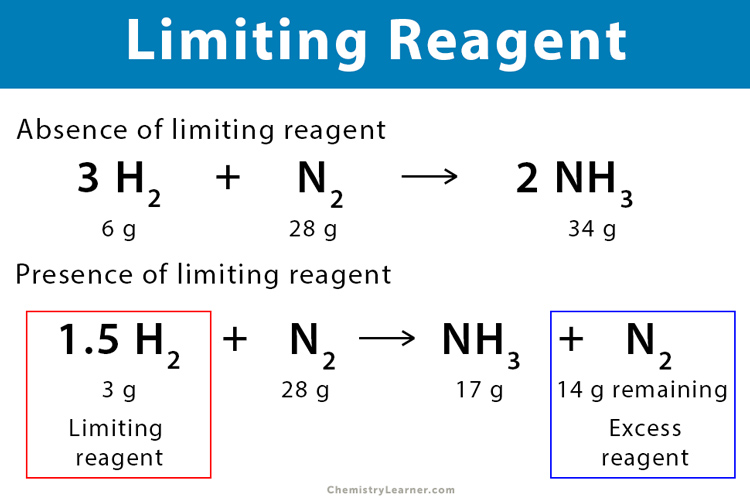
Excess Reactant Analysis
After identifying the limiting reactant, analyze the **excess reactant**. Knowing which reactant remains unreacted is pertinent not only for completeness but also for theoretical analyses in chemistry labs. Once you confirm the limiting factor, calculate how much of the other reactants will remain after the reaction completes. This is crucial for yields, as you might want to recycle excess
material for future reactions or adjust supplies accordingly.
Practical Applications of Stoichiometry
In practical applications, such as **laboratory investigations**, mastering stoichiometry can greatly enhance efficiency and accuracy. For example, knowing how to calculate yields accurately can save time and resources in an academic setting. It can also introduce you to advanced stoichiometry, where real-life implications of calculations lead to successful lab results and innovative advances in chemistry.
Common Mistakes in Stoichiometry
Even seasoned chemists can make mistakes in stoichiometric calculations. This section identifies common pitfalls to help you achieve proficiency in determining limiting reactants.
Common Errors in Reaction Yields
When working with **calculations in chemistry**, small numerical errors can lead to vastly different results. Although mistakes can happen, being mindful of units and ensuring that you double-check your work can prevent many errors, especially when determining limiting reactants. Another common issue is not properly balancing the **chemical equations**, which builds inaccuracies right from the start and skews the whole calculation. Remember: balanced reactions are your foundational blocks!
Identifying Reactants in Laboratory Settings
Another error arises from poorly understanding **reactant limitations** and deductions during experiments. Sometimes students confuse theoretical yields with actual outcomes. Familiarizing yourself with various methods for determining the limiting reactant ensures you have the right tools to succeed. For instance, the volumetric methods can be very useful for measuring solutions accurately. When taking measurements, always remember to calibrate instruments when conducting a lab experiment to limit discrepancies.
Using Online Tools for Better Results
With the advent of technology, utilizing **online stoichiometry calculators** can enhance effectiveness. Many platforms now offer interactive chemistry labs and online tutorials that simplify processes like calculating limiting reactants. These tools are beneficial for reinforcing classroom learning and providing immediate feedback for mastering concepts related to **finding limiting reactant steps**.
Key Takeaways
- Understanding limiting reactants is crucial for predicting reaction yields.
- Properly balanced chemical equations and mole ratios are foundational for stoichiometric calculations.
- Common mistakes in calculations often stem from unbalanced equations or measurement errors.
- Technological resources available offer valuable supports to students and professionals alike.
- Applying stoichiometric principles helps refine analytical techniques in the lab.
FAQ
1. What is the significance of limiting reactants in a chemical reaction?
The significance of limiting reactants in a chemical reaction lies in their role in determining the maximum amount of product that can form. Understanding which reactant will be exhausted first, referred to as the limiting reactant, enables chemists to project the **theoretical yield** of reactions accurately and optimize reactant use for economic and practical advantages.
2. How do I balance a chemical equation effectively?
To effectively balance a chemical equation, start by writing down the number of atoms of each element present on both sides of the equation. Adjust the **stoichiometric coefficients** in front of reactants or products until you achieve the same quantity of each type of atom on both sides. Always double-check! Tools are widely available to assist in balancing equations for immediate reinforcement of techniques learned.
3. Can excess reactants be recycled in future reactions?
Yes, excess reactants can often be recycled in future reactions, which can lower costs and improve overall resource efficiency. However, ensure that the quality and concentration of these recycled reactants are checked to prevent detrimental effects on new reactions. Understanding this process is also important for conducting comprehensive **reaction types** and stoichiometric evaluations.
4. What are the most common mistakes when calculating limiting reactants?
The most common mistakes involve failing to balance equations properly, using incorrect mole ratios, or making miscalculations in stoichiometric conversions, such as **mass-to-mole conversions**. It’s also essential to recognize and avoid premature conclusions drawn from incomplete data. Care and accuracy in calculations are paramount.
5. Where can I find additional resources for learning about limiting reactants?
Numerous resources are available online, including educational chemistry websites, video tutorials, and interactive experiments that reinforce principles of **stoichiometry** and **finding limiting reactants**. Sites like educational forums, open-source materials, and tools for interactive learning help facilitate deeper understanding, alongside practical examples from academic courses and chemistries on project ideas.
6. How does stoichiometry relate to chemical reaction efficiency?
Stoichiometry plays a significant role in **reaction yield** calculations by ensuring that the correct amounts of reactants are used. By knowing the limiting reactants, chemists can avoid wastage of materials and enhance the efficiency of reactions, ultimately saving time and resources during laboratory investigations.
7. What tools can I use to simplify stoichiometric calculations?
A variety of tools, including online stoichiometry calculators and interactive simulations, can simplify **stoichiometric calculations** significantly. Additionally, educational technology tools and resources can reinforce the understanding of the relationships between reactants and products, aiding in the learning process and practical applications in the laboratory setting.
By mastering the essential skills needed to identify and calculate limiting reactants, students and practitioners can greatly enhance their understanding of **chemical computations** and improve overall problem-solving strategies within the field of chemistry.