Effective Ways to Calculate Moles in 2025: Understand and Apply Key Concepts
Calculating moles is fundamental in chemistry as it bridges the gap between the macroscopic world of bulk materials and the microscopic world of atoms and molecules. Whether you’re working in a laboratory, studying for an exam, or simply curious about chemical reactions, mastering the **mole concept** is crucial. This article provides a comprehensive overview of effective ways to calculate moles, guiding you with practical tips and key concepts essential for quantitative analysis.
Understanding the Mole Definition and Importance
The **mole definition** serves as a cornerstone in chemistry, representing 6.022 x 10²³ particles, known as Avogadro’s number. This unit measures amounts of substances, ensuring consistency in **moles calculation** across different chemical contexts. Understanding how to quantify a substance through moles provides valuable insight into conducting laboratory experiments, performing chemical reactions, and interpreting results accurately. For example, knowing that one mole of sodium chloride contains Avogadro’s number of Na and Cl particles is essential for stoichiometric calculations.
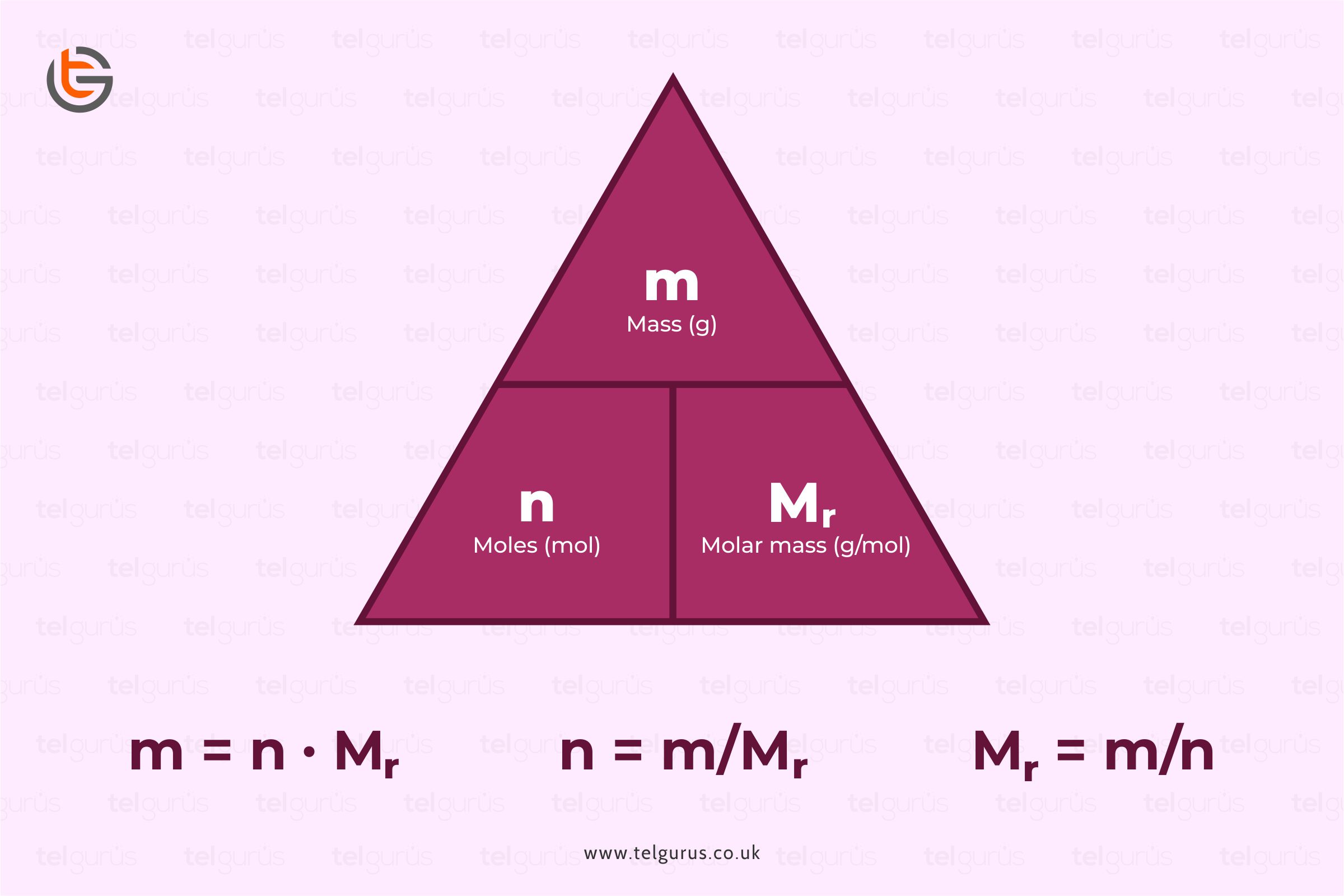
The Role of Molar Mass in Mole Calculations
Molecular weight, also known as molar mass, directly relates to how we calculate moles. By dividing the mass of a compound (in grams) by its **molar mass**, we can determine the number of moles present. For instance, if you have 58.44 grams of NaCl, the computation would be: moles = mass (g) / molar mass (g/mol). In this case, 58.44 g NaCl / 58.44 g/mol = 1 mole. This understanding is essential when preparing **standard solutions** for titrations in laboratories.
Applying Stoichiometry in Mole Calculations
**Stoichiometry** involves using balanced chemical equations to relate the moles of reactants to products. In any chemical reaction, the coefficients indicate stoichiometric ratios. For instance, in the combustion of methane (\(CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O\)), the mole ratio of \(CH_4\) to \(O_2\) is 1:2. This ratio assists in **conversions** during molarity calculations or identifying the **limiting reagent** in reactions. Therefore, conceptualizing and correctly applying stoichiometry ensures accuracy in chemical equation balancing and results interpretation.
Molarity and Concentration Calculations
**Molarity** (M) represents the concentration of a solute in a solution and is calculated using the formula: molarity = moles of solute / liters of solution. This concept is vital for understanding how concentrated a solution is, which influences the outcomes of chemical reactions. For instance, if you have 0.5 moles of NaCl in a 2-liter solution, the molarity would be 0.25 M. Understanding molarity aids in the preparation of buffer solutions and determining **reaction yield**.
Concentration Units and Their Application
In addition to molarity, several **concentration units** such as molal concentration and mole fraction play a significant role in chemistry. While molarity expresses solution concentration in terms of volume, molal concentration looks at the mass of solvent. This differentiation is essential when calculating **dilution formulas** or preparing solutions for **volumetric analysis**. For a practical example, to prepare a 1 molal (1m) sucrose solution, you would dissolve 342 grams of sucrose in one kilogram of water, emphasizing the significance of understanding various concentration measurements.
Understanding Solution Preparation and Dissolution Process
The **dissolution process** represents how solute particles disperse throughout a solvent. Successful solution preparation requires precise calculations combining the amount of solute and solvent. For instance, during **titration**, accurately calculating the moles of titrant used allows chemists to determine the concentration of an analyte present in the solution. By employing a step-by-step approach, such as calculating the molarity of the titrant or understanding the equivalence point, effective quantitative analysis can be achieved in laboratory settings.
Gas Laws and Their Applications
Understanding the relationship between moles and gases is revolutionized through various **gas laws**, particularly at standard conditions. The **ideal gas equation** (PV=nRT) sharply illustrates this relationship, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin. Employing this equation helps predict gas behavior under varying conditions. For instance, estimating the number of moles of gas in a stored natural gas tank through given pressure and volume illustrates real-world applications of this theory.
Volume Calculations in Chemistry
An essential application of the ideal gas equation is understanding **gas volume calculations**. Using the formula, if 2 moles of gas are occupying a volume of 22.4 liters at standard temperature and pressure (STP), one could rearrange the equation to extract any unknowns related to molar amounts and gas volumes. This knowledge is vital for practical scenarios involving gas reactions, where accurate volume and pressure data inform on the moles of reactants and products involved.
Empirical and Molecular Formulas: Understanding Chemical Identity
Determining empirical and molecular formulas is critical in identifying the composition of **chemical compounds**. The empirical formula denotes the simplest ratio of elements, while the molecular formula shows the actual number of atoms. For instance, ethanol’s empirical formula (C2H6O) depicts its most reduced state, while its molecular form is consistent for stoichiometric calculations relating to **reaction balances**. Cultivating proficiency in identifying these formulas strengthens one’s ability to conduct effective chemical analyses and apply mole calculations in various scenarios.
Key Takeaways
- The mole is a critical measurement in chemistry, representing Avogadro’s number of particles.
- Understanding molar mass is essential for accurate moles calculation and stoichiometry.
- Molarity connects solute amount to solution volume, crucial for concentration measurements.
- Gas laws, including the ideal gas equation, facilitate understanding of the behavior of gases in relation to moles.
- Mastering empirical and molecular formulas aids in determining chemical compositions and conducting accurate laboratory analyses.
FAQ
1. What is Molarity and How is it Calculated?
Molarity (M) quantifies a solution’s concentration as moles of solute per liter of solution. It is calculated using the formula: Molarity = moles of solute / liters of solution. For example, if you dissolve 1 mole of NaCl in a final volume of 1 liter of water, the solution has a molarity of 1M, indicating each liter contains one mole of the solute.
2. How Do I Calculate Moles from Mass of a Substance?
To calculate moles from mass, divide the mass of the substance by its molar mass. The equation is: moles = mass (g) / molar mass (g/mol). For instance, if you have 20 grams of glucose (molar mass ~180 g/mol), you would calculate 20 g / 180 g/mol = 0.111 moles of glucose.
3. What is the Importance of Avogadro’s Number?
Avogadro’s number (6.022 x 10²³) is crucial in chemistry as it defines the number of atoms, molecules, or particles in one mole of substance. It provides a bridge between the microscopic atomic scale and the macroscopic scale we experience, enabling chemists to relate moles to real-world measurements and calculations effectively.
4. How Do Stoichiometric Ratios Influence Chemical Reactions?
Stoichiometric ratios, derived from balanced chemical equations, influence reaction outcomes by defining the proportions of reactants required to produce a specific amount of product. These ratios guide calculations of limiting reagents, determining how much of the reactants will convert into the desired products during a reaction, ensuring comprehension throughout different chemical scenarios.
5. Can Gas Laws Help Predict Chemical Behavior?
Yes, gas laws like the ideal gas equation allow chemists to predict how gases behave under varying temperature and pressure conditions. This predictive ability is essential in laboratory settings and industrial applications, providing insight into the amounts of gases involved in reactions, compressibility, and real-time interaction under specific conditions.